Awaiting Word on Rescheduling, the Market Watches Epidiolex
By William Sumner, Hemp Business Journal Contributor
Pending word at any time, the United States Drug Enforcement Administration (DEA) is expected to reschedule Epidiolex, a CBD-based drug derived from cannabis. An announcement was due on Sept. 23, but by press time remained forthcoming.
Manufactured by the London-based GW Pharmaceuticals, the drug has been shown to be useful in the treatment of two forms of severe juvenile epilepsy.
It is unclear to what extent the DEA will reclassify Epidiolex. However, analysts at Morgan Stanley expect the agency to move the drug from a Schedule I substance to either a Schedule III or IV substance; the latter would categorize the drug similarly to codeine or Xanax.
Epidiolex was initially approved for medical use by the U.S. Food and Drug Administration (FDA) last June, making it the first CBD-based drug, and the first cannabis-based drug to gain FDA approval. Under federal law, the DEA is required to reschedule drugs within 90 days of FDA approval.
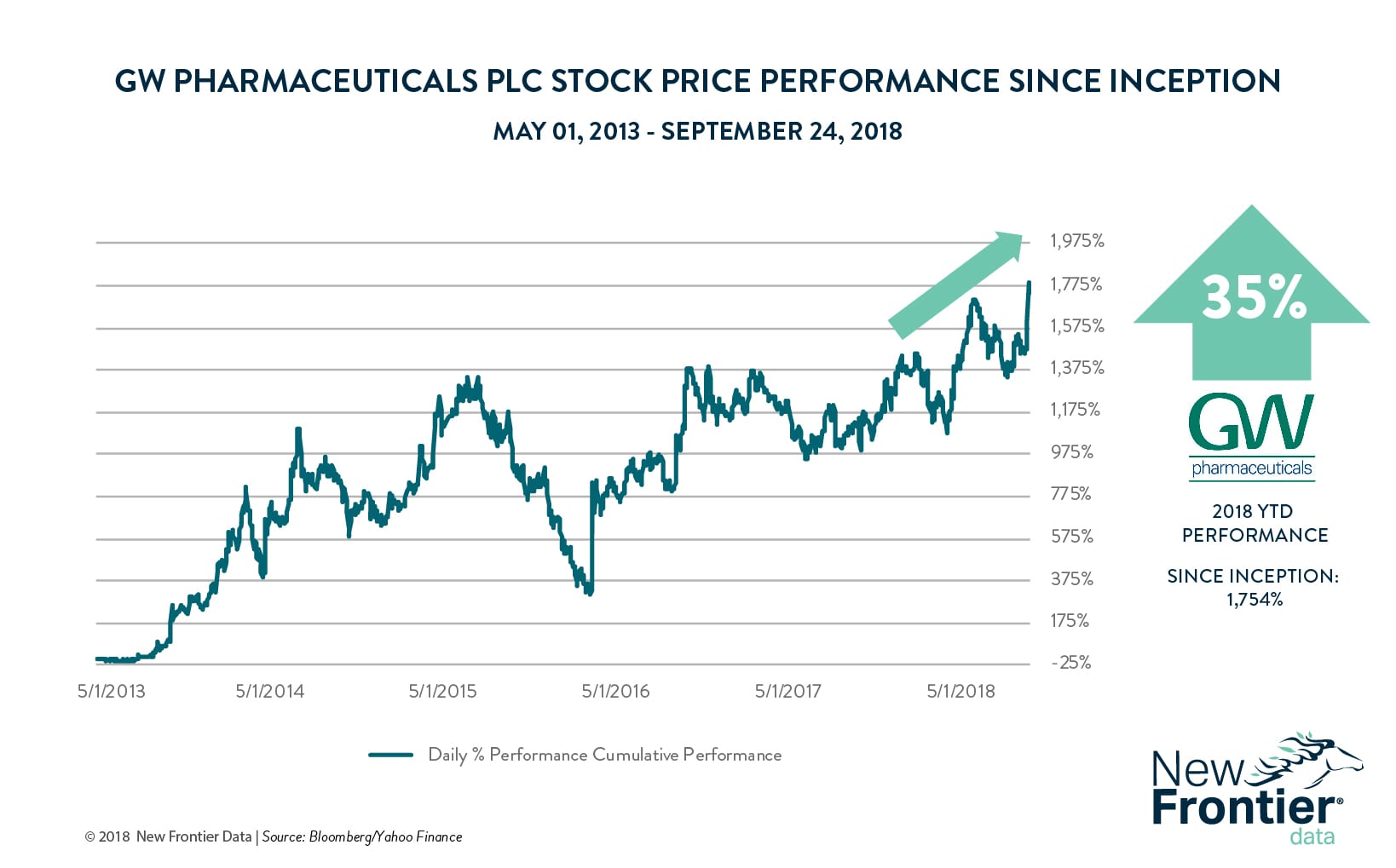
In response to the impending decision, GW Pharmaceuticals’ stock has surged over the last 10 days, rising 17% from $141.18 on Sept. 14 to $166.52 at the close of business on Sept. 24. Since going public in 2013, GW’s stock has risen exponentially; growing by at least 1,775% since its inception, and roughly 35% since the start of this year.
Although Epidiolex is derived from cannabis, it is unlikely that much will change with regards to federal cannabis policy. Earlier this year, the DEA reported that it currently has no intentions on rescheduling cannabis.While the DEA did note how that pertained to the rescheduling of CBD itself, deviation seems unlikely since individual drugs often receive their own scheduling classification.
Once on the market, Epidiolex is expected to cost approximately $32,500 per year, in keeping with the price of other epilepsy-related medicines such as Fycompa, which costs roughly $1,500 a month.
For patients with either private insurance or Medicaid, the price is expected to fall precipitously. According to the Wall Street Journal, Medicaid recipients can expect to pay between $5-$10, while patients with insurance could spend upwards of $200 a month.
The Hemp Business Journal estimates that Epidiolex could generate between $15 million to $30 million in sales annually. After the drug is approved in Europe, which is expected to happen in the first quarter of 2019, European sales are expected to generate approximately $215 million by 2022.


William Sumner
William Sumner is a writer for the hemp and cannabis industry. Hailing from Panama City, Florida, William covers various topics such as hemp legislation, investment, and business. William’s writing has appeared in publications such as Green Market Report, Civilized, and MJINews. You can follow William on Twitter: @W_Sumner.